

Thirty years on, we are very well positioned in this field and can offer our customers both traditional Sanger sequencing services as well as state-of-the- art next generation sequencing services. In 1992, Microsynth decided to enter the field of DNA sequencing. We now offer our numerous customers a broad spectrum of high-quality DNA/RNA oligonucleotides for research, drug development and molecular diagnostics. Schmidheini started producing DNA oligonucleotides on behalf of Swiss academic institutes, he was one of the pioneers in this field. Tobias Schmidheini as a spin-off of ETH Zurich.
MICROSYNTH AG ISO
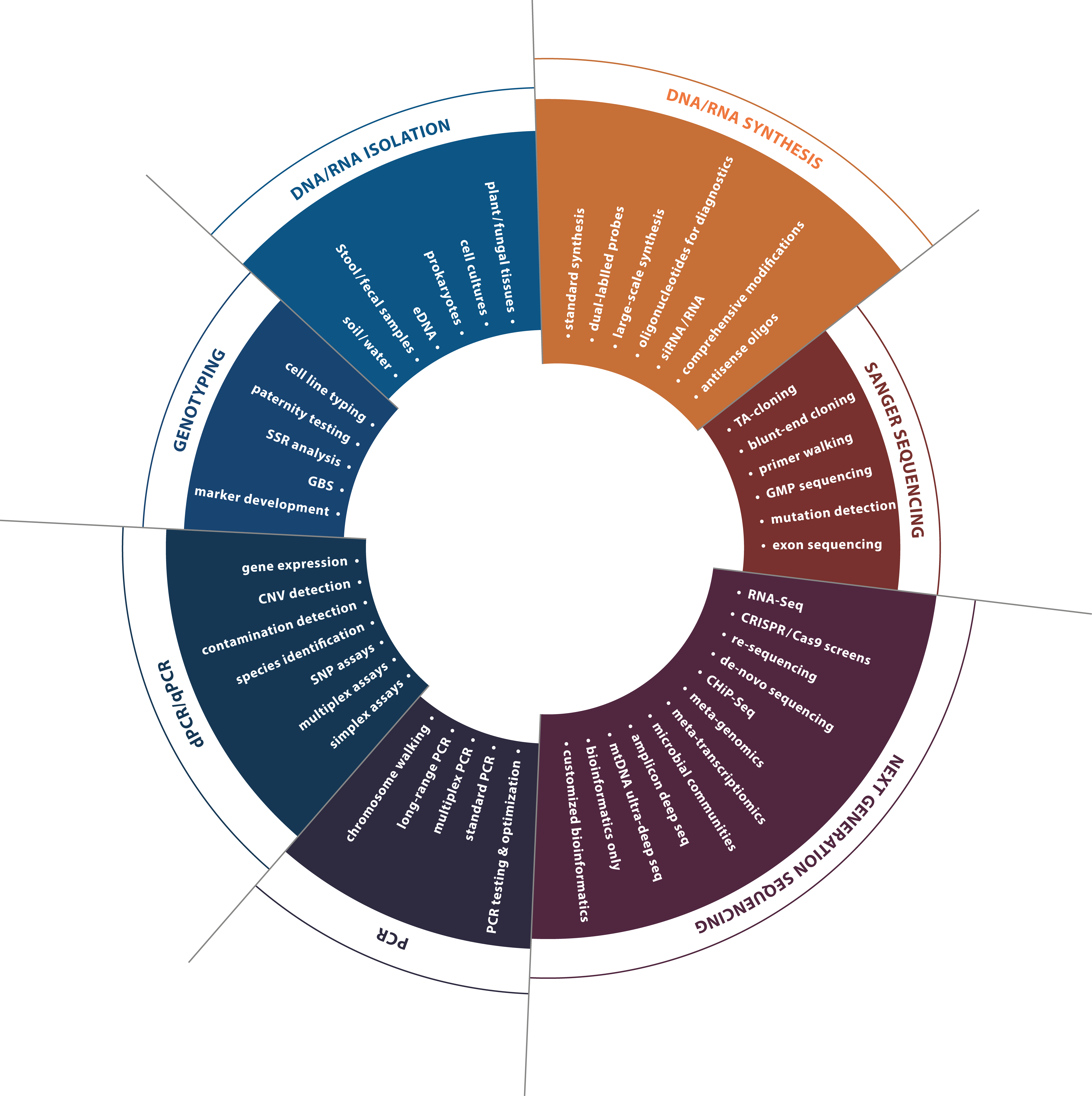
The oligonucleotide synthesis of Microsynth AG (Balgach) is EN ISO 13485:2016 certified for the production and distribution of nucleic acids and components for IVD manufacturers and provision of associated activities. Further, Microsynth AG (Balgach) is authorized by SwissMedic to perform quality control of medicinal products by GMP Sanger sequencing. The services of Microsynth AG (Balgach) related to NGS and Sanger sequencing as well as to fragment length analysis are additionally ISO/IEC 17025:2017 accredited (STS 0429). Microsynth AG, Microsynth Seqlab and Microsynth Austria are ISO 9001:2015 certified. Altogether Microsynth employs a staff of > 100 people. Microsynth has subsidiaries in Germany (Microsynth Seqlab GmbH), Austria (Microsynth Austria GmbH), France (Microsynth France SAS), and Switzerland (ecogenics GmbH). The main activities are divided into following three business areas:įor more than three decades, the company’s objective has been to serve its customers by delivering products and services of the highest quality, on time and with outstanding service – and all this at competitive prices. Microsynth (founded in 1989) is a leading European company in nucleic acid synthesis & analysis.


 0 kommentar(er)
0 kommentar(er)
